The art and science of formulating
In a new step-by-step guide, Stability Testing Services – a division of Botanichem – explores the various stages of product development.


Image: Botanichem
Image: Botanichem
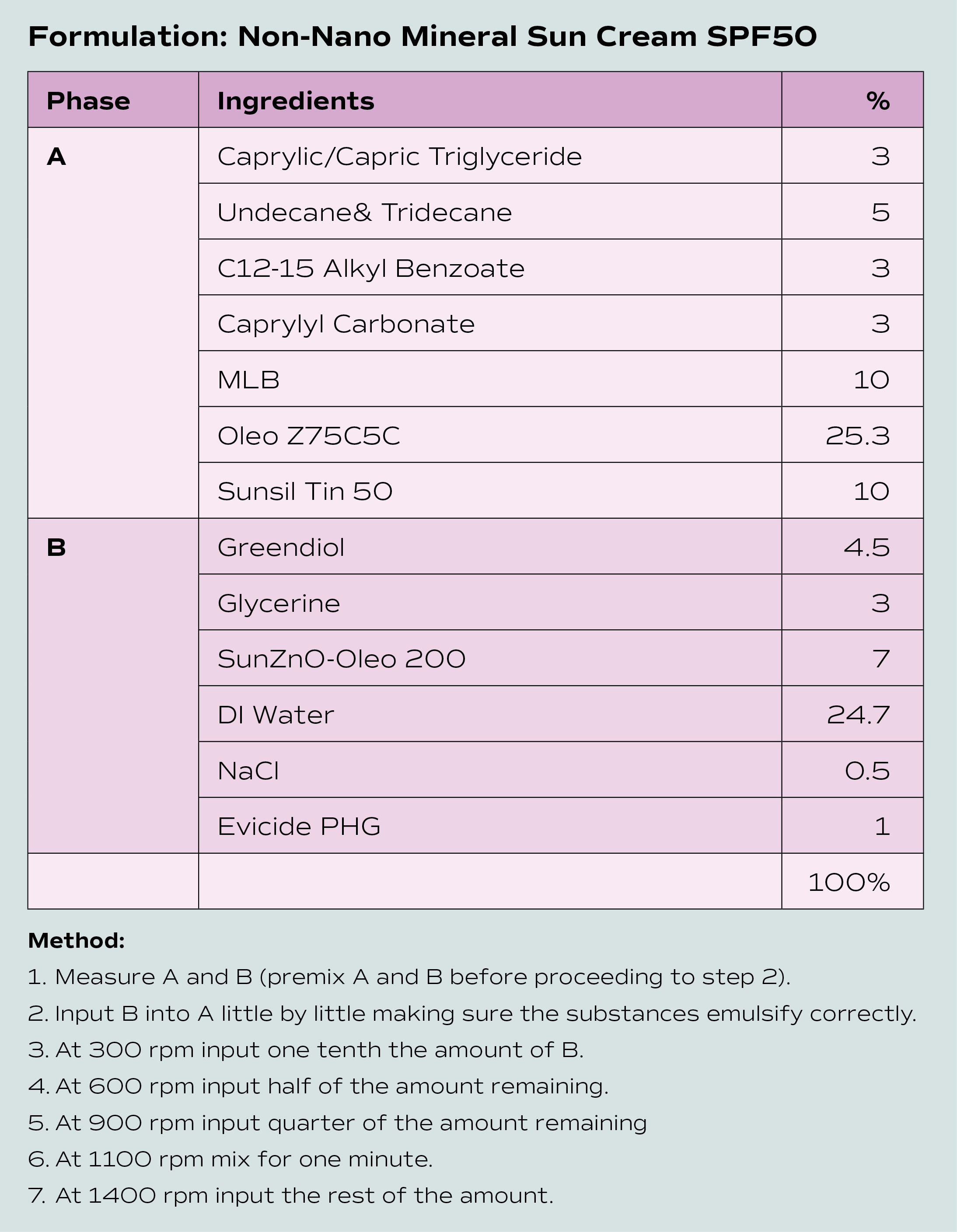

Image: Shutterstock
Image: Shutterstock
Formulating is a meticulous and methodical process, which depends on the complexity and number of products being developed. It can take between nine and 24 months (or often longer) to complete. Formulation development involves several steps, all of which are critical to the ultimate success of the product.
“Formulation is part art, part science,” says Robyn Brown, owner of Stability Testing Services. “The scientific aspect is the heart of the process. As formulators we have an in-depth knowledge of ingredients, how they combine, what quantities to use and why to include them. The art side of it is being able to take this knowledge and the client’s brief and create the perfect product that matches their expectations.”
1. Market research and analysis (duration – one to two months)
This is not necessarily something undertaken directly by the formulator although they can advise and provide important insight into market trends especially when it comes to ingredients. The aim of this is to determine whether the product addresses a genuine gap or need in the market.
2. Concept development and ingredient selection (duration – one to two months)
Based on the market research, a concept for the product is established. This includes defining the target audience, the product's purpose (e.g., anti-ageing, hydration, cleansing), and its desired benefits. Ingredient selection then needs to take place.
3. Initial formulation and stability testing (duration – four to six months)
The formulator will create a prototype using the chosen ingredients. This can be quite a lengthy process as texture, stability and efficacy need to be considered.
Specialised lab equipment is used to measure factors like pH level, viscosity and microbial growth. It often takes several samples before arriving at one that the client is happy with. Once the client approves the formulation, a sample batch of the product is made and stability tests are done to assess how the product performs over time under various conditions (like temperature and light exposure) to ensure its shelf life and safety. At this stage packaging also needs to be finalised to ensure compatibility with the product. It is advisable to conduct stability tests in the final packaging to ensure the product is compatible with its packaging.
4. Preservative selection and challenge testing (duration – one to two months)
Preservatives are crucial to prevent microbial growth and extend shelf life. Preservatives are selected based on compatibility with ingredients and their effectiveness against specific microbes. Challenge testing involves exposing the product to potential contaminants under extreme conditions to assess the efficacy of the chosen preservatives.
Quality and safety cannot be compromised as this will affect the long-term success of the product
6. Regulatory compliance (duration – one month)
There are stringent regulations regarding labelling. Labels need to include ingredient listing (using INCI names), usage instructions, expiry dates and manufacturing details. This is particularly important if you are planning to sell your product commercially or export it.
7. Manufacturing scale-up (duration – three to four months)
Once all approvals are obtained, the formulation is adapted for large-scale production. Manufacturing processes are also established now to ensure consistency and quality control. Another stability test needs to be performed at this stage to ensure that the scale up batch is stable before manufacturing the final batch. If it isn’t, tweaks to formulation might be required.
Dealing with challenges
Whilst every effort is taken to streamline the product development process, issues may arise and often this means repeating a particular step which will delay the process. Ultimately quality and safety cannot be compromised as this will affect the long-term success of the product.
For more information on formulation development and stability testing, send an email to robyn@botanichem.co.za.










