Original study
Treatment outcomes with micropulse transscleral cyclophotocoagulation in refractory and non-refractory glaucoma in a South African context: a retrospective analysis of 163 eyes
N Narainswami MBBCH (Wits), Dip Ophth (SA), Dip HIV Man (SA), FCP Ophth (SA), MMED (UKZN), Fellow in Paediatric Ophthalmology and Strabismus Red Cross War Memorial Children’s Hospital, Greys Hospital, Pietermaritzburg, KwaZulu-Natal, South Africa.
ORCID: https://orcid.org/0000-0002-5495-7153
C Kruse FCOphth, MMed(Ophth), Head of Department University of KwaZulu-Natal Ophthalmology, Durban, KwaZulu-Natal, South Africa.
ORCID: https://orcid.org/0000-0002-8805-8383
Corresponding author: Dr Neeran Narainswami, e-mail: neerannarainswami@gmail.com

Introduction
Sub-Saharan Africa has the highest prevalence and incidence of glaucoma globally.1,2 Late presentation coupled with suboptimal treatment options and difficult follow-up remains a problem for this ‘silent thief of sight’ in South Africa.1
Modern trends of earlier surgical intervention coupled with the development of new micro surgical options holds fresh promise. Micropulse trans-scleral cyclophotocoagulation (M-TSCPC) is used successfully in Europe and USA as a novel primary treatment option.3-6
In recent years there has been a surge in novel glaucoma surgical interventions. Currently the ‘gold standard’ for surgical intervention remains augmented trabeculectomy or tube shunt in advanced or rapidly progressive glaucoma.7 The attendant complication rates of incisional ocular surgery, however, as well as unrealistic patient expectations for an operation that does not improve vision but merely delays progression of visual loss, have meant that ophthalmologists the world over have been in search of the elusive ‘holy grail’ of glaucoma surgery that is safe, cost-effective and sight saving. This study seeks to find whether M-TSCPC is effective in the treatment of a various types of glaucoma and can become a valuable tool in our treatment armamentarium.
Glaucoma is the second leading cause of preventable blindness globally2 and primary open angle glaucoma (POAG)predominates in African populations such as those found in sub-Saharan Africa.8,9 It is also well recognised that African patients tend to have a more aggressive disease course with higher intraocular pressures, earlier age of onset and blindness.8,9 Treatment of this insidious cause of irreversible blindness remains a challenge, as presentation is often delayed with up to 50% of patients having already completely lost vision in one eye on first presentation.8,9 Population-based surveys carried out in South Africa, Tanzania and Ghana show that the overwhelming majority of glaucoma patients at diagnosis were not even aware they had the disease.9 Glaucoma also remains a poorly understood disease by patients themselves which further compounds matters. Medical treatment, which entails daily topical anti-glaucoma drops, is costly, and oral acetazolamide with its unpleasant side-effect profile further exacerbates poor adherence.
The surgical outcomes of augmented trabeculectomy are not always reproducible even in the same hands and the postoperative course requires frequent and meticulous follow up, which is rarely feasible in resource poor settings.7,8 This problem is further highlighted by the fact that 80% of the South African population lives in rural areas while the vast majority of qualified ophthalmologists practice in urban centres.8
Historically, continuous wave transscleral cyclophotocoagulation (CW-TSCPC) has been relegated to treating poor prognosis glaucomatous eyes by means of ablation of the ciliary body to decrease aqueous humour production and thus lower intraocular pressure.4,5 Cyclocryotherapy, the original cyclodestructive procedure, is increasingly being supplanted by laser cyclophotocoagulation at first with the neodymium:YAG laser (1064nm) and further with the 810nm continuous wave diode laser.10
While modern day incisional glaucoma surgery is geared towards increasing aqueous outflow from the eye to lower intraocular pressure, transscleral cyclophotocoagulation acts to decrease aqueous production by ablating the ciliary epithelium.10 Most published studies only include eyes with initial poor visual function, mostly due to advanced and usually severe refractory types of glaucoma such as uveitis and neovascular glaucoma.4,5,10-12 In recent years there have been many refinements in the laser systems which are available today for cyclophotocoagulation, such that these complications have become a rarity.13-15
The advantages of M-TSCPC over conventional CW-TSCPC lies in its novel technology. The continuous wave of laser energy is now chopped up into ‘on’ and ‘off’ delivery cycles that allows the target tissue recovery time to ‘cool down’ and prevents thermal-induced structural damage to the ciliary body, while inducing biological reactions selectively within the pigmented ciliary epithelium.11,16,17 The ciliary epithelium is able to regenerate and multiple repeat treatments can be performed to induce a long-term hypotensive state while minimising the risks of phthisis bulbi, sympathetic ophthalmia and ocular hypotony seen more commonly with older cycloablative procedures.17-19
Materials and methods
This is a retrospective chart review of all patients undergoing M-TSCPC at Grey’s Hospital Eye Clinic from January 2017 to December 2019 (three years). Patients with missing records or without a minimum of six months follow- up post laser were excluded. Patients with refractory (inadequate IOP control despite maximal medical therapy) and non-refractory glaucoma with good vision were included. Data collected included demographic data as well as baseline pre-treatment IOP, IOP at one month, two months, three months and six months post treatment, number of medications used, visual acuity at each visit and any reported side effects. The type of glaucoma and number of prior glaucoma surgeries was also documented. The procedure was performed in the outpatient clinic under peribulbar local anaesthesia with resuscitation equipment on hand. Each treatment session consisted of 160 seconds of 2000MW 810nm laser in a 31.3% ‘on’ cycle, using the Iridex® Cyclo G6® Glaucoma Laser System together with the MP3 curved probe for 360 degrees. Areas of obvious scleral thinning and at 3 and 9 o’ clock were avoided. Repeated treatments were titrated to response with a maximum of four treatments per eye. No generalised fixed target pressure was used. Instead, retreatment was done in eyes with suboptimal IOP lowering according to each individual patient’s target IOP, and/or in cases where the medication load was deemed too high (especially patients on chronic oral acetazolamide). The same team of doctors carried out all treatments. All patients continued their regular antiglaucoma medication after laser and were given topical prednisolone acetate 1% drops two-hourly for at least two days and then tapered over two weeks.
Medians and Inter-Quartile Range (IQR) were used for non-normal distribution. Wilcoxon-Mann-Whitney paired test was used to compare the pre- and post-treatment intraocular pressures. Subgroup comparisons by type of glaucoma and race was examined using ANOVA and Kruskal-Wallis statistical tests. The success rate in the various groups was compared using a Chi Square test. Primary outcomes measured were reduction in IOP and reduction in medication usage. Secondary outcomes included any complications reported and a decline in mean visual acuity from baseline.
Success was defined as a 20% reduction in IOP with or without medication and any decrease in glaucoma medication usage. Data was analysed in Stata V13 and a p-value of 0.05 or less was considered statistically significant.
Ethical approval was obtained from the University KwaZulu-Natal bioethics research committee (BREC).
Results
163 eyes of 137 patients were treated with M-TSCPC at least once in the three-year period. The total number of treatments given was 265. The baseline demographics are shown in Table I: The mean age was 57 years (SD = 15), 57% (n = 77) were male and the majority were Black (n = 109) patients.
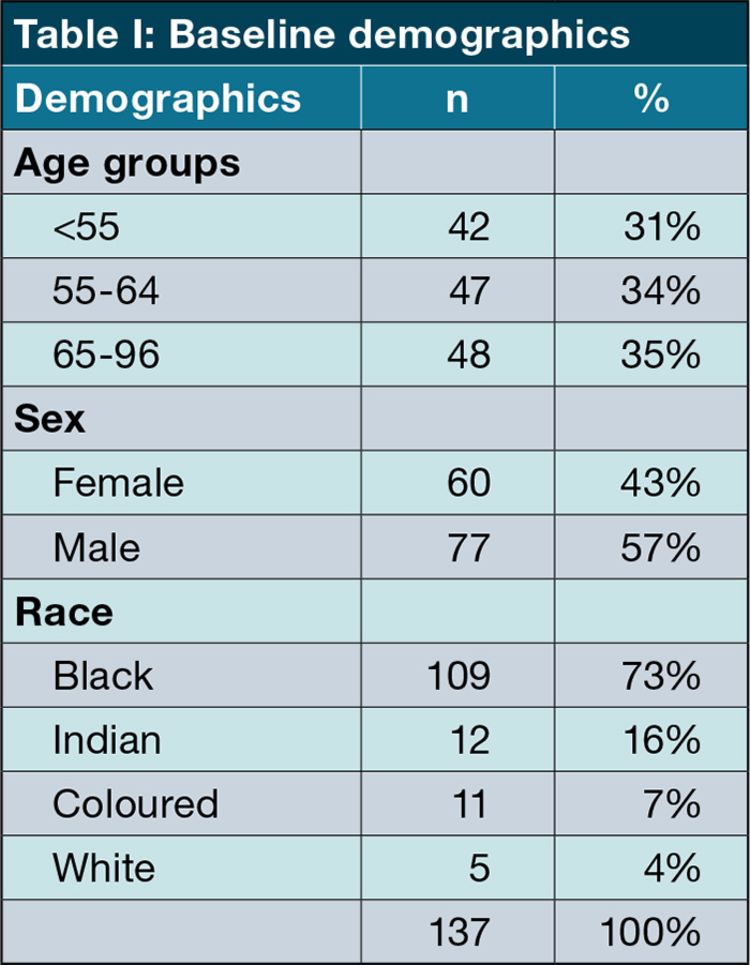
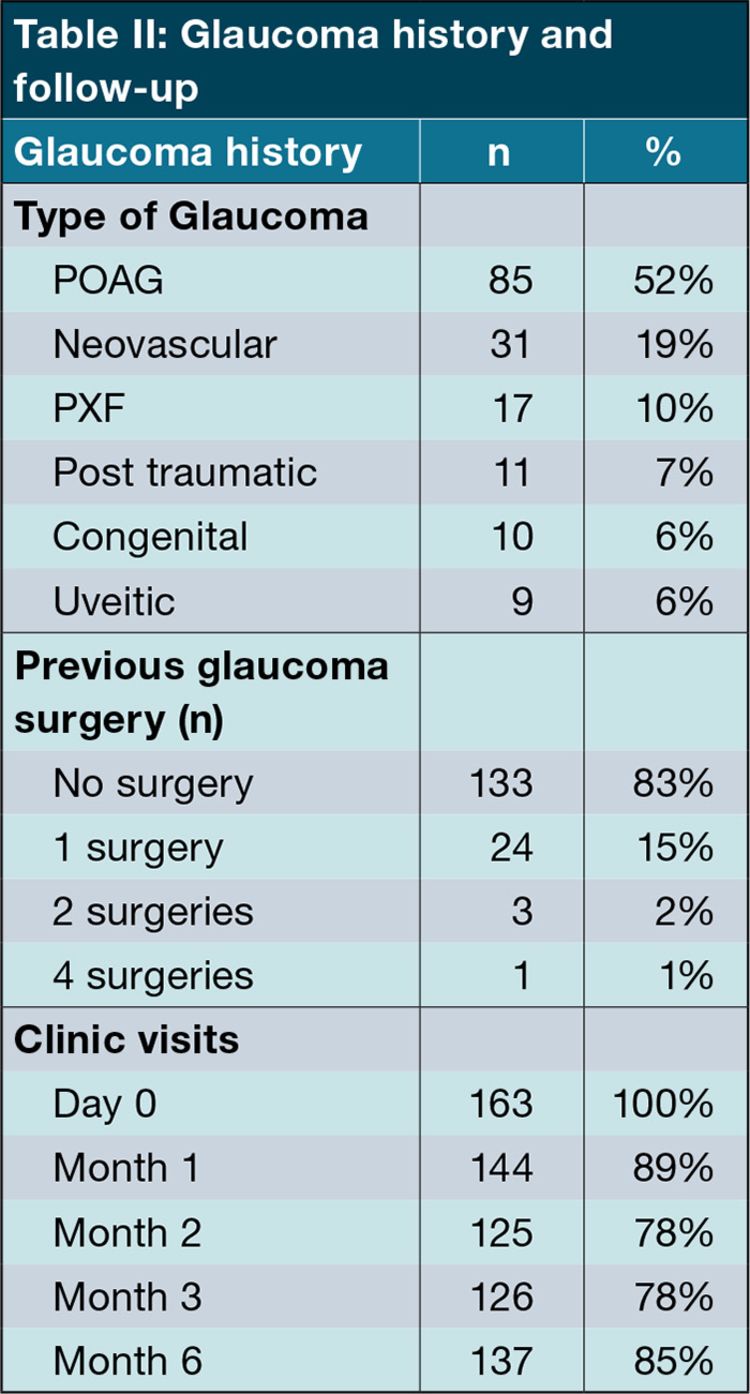
Table II shows an overview of the glaucoma history of the patients. The most common types of glaucoma were primary open angle glaucoma (POAG: 52%, n = 85) and neovascular glaucoma (19%, n = 24). 18% (n = 24) of eyes had had at least one previous glaucoma surgery before the first M-TSCPC treatment. 137 eyes (85%) were followed up to the six-month visit.
Mean intraocular pressure dropped from 38 mmHg (SD = 12.8) to 22 mmHg (SD = 11.8) by the first month after treatment (p<0.001). Figure 1 shows how the mean IOP continued decreasing to 17 mmHg (SD = 7.7) at month six (p<0.001 from baseline).
Figure 1: Mean IOP from baseline up to six months. The first treatment occurred directly after the baseline IOP had been measured.
Figure 1: Mean IOP from baseline up to six months. The first treatment occurred directly after the baseline IOP had been measured.
Overall glaucoma medication use dropped from 3.57 (SD = 0.54) agents at baseline to 2.51 (SD = 1.02) at month six (p<0.001). Seventy two percent of patients on oral acetazolamide (n = 66 of 92) were able to stop this chronic medication.
Neovascular glaucoma was the only subtype of glaucoma that showed a significant change in IOP decrease (-35 mmHg) compared to the reduction seen in POAG (-14.5 mmHg) patients as shown in Table III (p = 0.001).
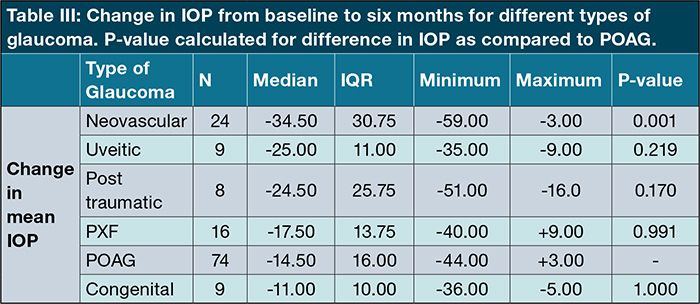
The factors that did not have a significant impact on IOP reduction were the number of previous glaucoma surgeries (p = 0.205) and number of pre-treatment glaucoma drops (p = 0.215).
Eyes with good or ambulatory pre-laser vision (VA> 0.1, n = 70) showed an initial slight drop in VA at month one of 0.11 (p = 0.045). By month six however, there was a return to baseline VA (p = 0.850 from baseline).
The main reported side effects in this study included discomfort (10%, n = 16), uveitis (7%, n = 11) and decrease in vision (3%, n = 5).
Discussion
There are at present no South African studies in the literature describing the effectiveness of M-TSCPC in eyes with good vision. In this study we examined the safety and effectiveness of M-TSCPC in a wide variety of glaucoma patients at different stages of disease and in seeing as well as blind eyes.
Earlier international studies have proved the efficacy of M-TSCPC as a valid treatment option much earlier in glaucoma management even in non-refractory glaucoma. A retrospective study by Ansari et al. which included eyes with good ambulatory vision (VA > 0.1) showed successful IOP lowering in 91% (n = 23) of patients in the POAG subgroup, with no decrease in mean VA at one year.19 All patients in this study from the United Kingdom were Caucasian. Varikuti et al. conducted an analysis of 61 eyes with good vision (VA>0.1 at baseline) and followed up for at least 10 months post M-TSCPC. 20.8% (n = 10) of eyes treated had two lines of vision loss at one year.20 Vision loss was mostly related to cataract progression and therefore amenable to treatment. Despite the negative association of visual loss that often haunts traditional TSCPC, visual loss is by no means the sole prerogative of any specific surgical option. The tube versus trabeculectomy study showed a 2 or more Snellen loss of vision in 33% of the augmented trabeculectomy group and 32% in the tube group after one year.7
A decrease in vision from baseline of 2 or more Snellen lines was documented in 3% of treated eyes in our study. This was attributed to progression of cataract (a reversible cause of vision loss) in four eyes (VA 0.5 to 0.2, 0.7 to 0.5, 0.7 to 0.3, 0.5 to 0.3) of patients ranging 68 to 75 years of age. One patient developed vitreous haemorrhage from central retinal vein occlusion. An IOP spike between baseline and the month one visit cannot be excluded but the final IOP was 11 mmHg (baseline 35 mmHg) with a drop of VA from 0.6 to 0.2. Of note this patient had defaulted all medication post treatment. No treated eyes became blind in our study.
Subgroup analysis (n = 70) of patients with good ambulatory pre-laser vision (VA> 0.1) showed an initial slight drop in VA at month one of 0.11 (p = 0.045). By month six however, there was a return to baseline VA (p = 0.850 from baseline). VA essentially remained the same in patients with good pre-laser baseline vision with no significant loss of vision. This could be attributed to the transient post-laser uveitis complicating 7% (n = 11) of eyes. Uveitis resolved completely in all patients.
Our study showed a mean reduction in IOP for all treated eyes from a baseline of 38.1 mmHg to 17.5 mmHg at six months, a drop of 54%. The neovascular glaucoma subgroup (n = 24) showed the greatest reduction in mean IOP by 34.5 mmHg over the six months follow up. The significant IOP lowering in these complex glaucoma group is encouraging and suggests that M-TSCPC may be used effectively in these otherwise difficult to manage patients. In comparison V A de Vries et al. showed a mean reduction in IOP of 31.8% (8.2 mmHg SD7.9) at six months using similar laser power settings (2000MW x 160 secs for 360 degrees) in his prospective cohort of 86 patients with a subgroup of 13 neovascular glaucoma (n = 14) eyes.6 All patients were Caucasian in this study.
The Early Manifest Glaucoma Treatment Trial found that on average each 1 mmHg reduction in IOP equated to 10% reduction in visual field loss.21 The POAG group in our study showed a mean reduction in IOP by 14.5 mmHg (p<0.001).
The success in reducing IOP in this study has been notable. Although not all patients had complete success, Figure 2 emphasises the improvement that was shown in a large majority of patients: 51% (n = 72) of eyes had an IOP reduction of over 40% at one month (p<0.001). At six months a reduction of IOP by least 30% occurred in 79% of eyes (n = 110), and a reduction of at least 20% in 92% (n = 129) of eyes (all p<0.001). An inherent weakness in this study is that we could not apply the descriptors ‘complete success’ nor ‘qualified success’ as defined in the WGA guidelines, as almost all patients continued at least one IOP-lowering medication post-treatment.
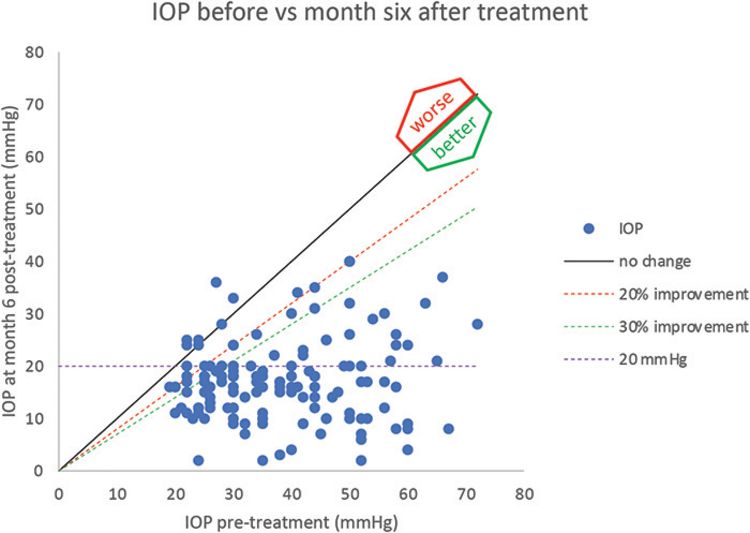
Figure 2: Comparison of IOP at baseline vs. 6 months after first treatment (n=137). All data points below the oblique solid line are reductions in IOP.
Figure 2: Comparison of IOP at baseline vs. 6 months after first treatment (n=137). All data points below the oblique solid line are reductions in IOP.
The safety and side-effect profile borne out in our study was reassuring and in keeping with prior studies.6,19,20 Ansari et al. reported a complication rate of 13% with hyphaema (n = 2), uveitis (n = 1). There were no cases of phthisis bulbi nor persistent hypotony as was also reported by Frezzotti et al. in his prospective long term follow up study of transscleral cyclophotocoagulation in 124 eyes with advanced refractory glaucoma.5,19 Kaba et al. also reported no persistent inflammation, hypotony nor sympathetic ophthalmia in 399 M-TSCPC treatments followed up for at one year in eyes which included ocular hypertension and normal-tension glaucoma.3 It is also interesting to note that this was despite higher laser power being employed in this study, of up to 2800MW. A recent local study by Manyeruke et al. in 24 refractory and blind glaucomatous eyes also reported no serious complications post M-TSCPC.22 Post-laser discomfort was the most common complication (10% n = 16). In our study; however, this discomfort was transient and was successfully managed in all patients by oral analgesics and topical anti-inflammatory drops. Although no cases of phthisis bulbi had developed, six eyes (5%) had an IOP of less than 5 mmHg at six months. Five of these were blind at baseline. At 12 months post treatment the seeing eye (VA 0.2) had an IOP of 19 mmHg off acetazolamide. IOP had improved to 6 mmHg in one of the blind patients at nine months. There were neither cases of sympathetic ophthalmia nor prolonged or recurrent uveitis. The majority of patients had mild to no side effects.
Seventy two percent of patients had stopped oral acetazolamide by month six and no longer required oral agents to maintain target IOP. Overall glaucoma treatment burden decreased from 3.57 to 2.51 medications.
What is evident in the literature when comparing outcomes of different protocols for M-TSCPC is that laser power (total energy delivered) during treatment appears to correlate with effectiveness of IOP lowering and complication rates.6 Our study had fewer complications than studies employing similar laser protocols.6,19 A prospective RCT would provide further elucidation on ideal fluence values required at different stages or types of glaucoma and identify any confounding variables.
Conclusion
M-TSCPC appears to be a safe and effective treatment modality in blind as well eyes with good vision. Successful IOP reduction of at least 20% was seen in 92% of treated eyes six months post laser. Treatment burden, especially with respect to oral agents, also decreased. This ‘cyclo-analogue’ to SLT seems an attractive option, especially for indigent glaucoma patient adherence and treatment effectiveness in South Africa.
References
- Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262-267.doi:10.1136/bjo.2005.081224.
- Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121:2081-2090.
- Kaba Q, Somani S, Tam E, Yuen D. The Effectiveness and Safety of Micropulse Cyclophotocoagulation in the Treatment of Ocular Hypertension and Glaucoma. Ophthalmol Glaucoma. 2020 May-Jun;3(3):181-189. doi: 10.1016/j.ogla.2020.02.005. Epub 2020 Feb 28. PMID: 32672613.
- Schlote T, Derse M, Rassmann K, Nicaeus T, Dietz K, Thiel HJ. Efficacy and safety of contact transscleral diode laser cyclophotocoagulation for advanced glaucoma. J Glaucoma. 2001;10(4):294-301. doi:10.1097/00061198-200108000-00009.
- Frezzotti P, Mittica V, Martone G, et al. Longterm follow-up of diode laser transscleral cyclophotocoagulation in the treatment of refractory glaucoma. Acta Ophthalmol (Copenh). 2010;88(1):150-155. doi:10.1111/j.1755-3768.2008.01354.
- V.A de Vries,Jan Pals, Hubb J Poelman et al. Efficacy and Safety of Micropluse Transscleral Cyclophotocoagulation. J Clin Med. 2022 Jun; 11(12): 3447. 10.3390/jcm11123447.
- Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153(5):789-803.e2. doi:10.1016/j.ajo.2011.10.026 .
- Scott D Lawrence, MD et al. Meeting the Challenge of Glaucoma in Africa. Glaucoma Today. https://glaucomatoday.com/articles/2013-july-aug/meeting-the-challenge-of-glaucoma-in-africa.
- Kyari F, Abdull MM, Bastawrous A, Gilbert CE, Faal H. Epidemiology of glaucoma in sub-Saharan Africa: prevalence, incidence and risk factors. Middle East Afr J Ophthalmol. 2013;20(2):111-125. doi:10.4103/0974-9233.110605.
- Aquino MCD, Barton K, Tan AMWT, et al. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: a randomized exploratory study. Clin Experiment Ophthalmol. 2015;43(1):40-46. doi:10.1111/ceo.12360.
- Schlote T, Derse M, Zierhut M. Transscleral diode laser cyclophotocoagulation for the treatment of refractory glaucoma secondary to inflammatory eye diseases. Br J Ophthalmol. 2000;84(9):999-1003. doi:10.1136/bjo.84.9.999.
- Martin KR, Broadway DC. Cyclodiode laser therapy for painful, blind glaucomatous eyes. Br J Ophthalmol. 2001;85(4):474-476. doi:10.1136/bjo.85.4.474.
- Meyer JJ, Lawrence SD. What’s new in laser treatment for glaucoma? Curr Opin Ophthalmol. 2012;23(2):111-117. doi:10.1097/ICU.0b013e32834f1887.
- Reconsidering Transscleral Cyclophotocoagulation. Glaucoma Today. Accessed December 20, 2022. https://glaucomatoday.com/articles/2012-jan-feb-insert/reconsidering-transscleral-cyclophotocoagulation.
- Holz HA, Lim MC. Glaucoma lasers: a review of the newer techniques. Curr Opin Ophthalmol. 2005;16(2):89-93. doi:10.1097/01.icu.0000156991.52256.56.
- Kuchar S, Moster MR, Reamer CB, Waisbourd M. Treatment outcomes of micropulse transscleral cyclophotocoagulation in advanced glaucoma. Lasers Med Sci. 2016;31(2):393-396. doi:10.1007/s10103-015-1856-9.
- Kaushik S, Pandav SS, Jain R, Bansal S, Gupta A. Lower energy levels adequate for effective transcleral diode laser cyclophotocoagulation in Asian eyes with refractory glaucoma. Eye Lond Engl. 2008;22(3):398-405. doi:10.1038/sj.eye.6702653.
- Rotchford AP, Jayasawal R, Madhusudhan S, Ho S, King AJ, Vernon SA. Transscleral diode laser cycloablation in patients with good vision. Br J Ophthalmol. 2010;94(9):1180-1183. doi:10.1136/bjo.2008.145565.
- Ansari E, Gandhewar J. Long-term efficacy and visual acuity following transscleral diode laser photocoagulation in cases of refractory and non-refractory glaucoma. Eye Lond Engl. 2007;21(7):936-940. doi:10.1038/sj.eye.6702345.
- Varikuti VNV, Shah P, Rai O, Chaves AC, Miranda A, Lim BA, Dorairaj SK, Sieminski SF. Outcomes of Micropulse Transscleral Cyclophotocoagulation in Eyes With Good Central Vision. J Glaucoma. 2019 Oct;28(10):901-905. doi: 10.1097/IJG.0000000000001339. PMID: 31385915.
- Leske MC, Heijl A, Hyman L, Bengtsson B. Early Manifest Glaucoma Trial: design and baseline data. Ophthalmology. 1999 Nov;106(11):2144-53. doi: 10.1016/s0161-6420(99)90497-9. PMID: 10571351.
- Manyeruke S, Tinley C, Du Toit N. The effectiveness and safety of micropulse transscleral cyclodiode photocoagulation therapy in glaucoma patients at Groote Schuur Hospital, Cape Town, South Africa. SA ophthalmol.J. 2022 Aug 1;17(3):16-20.