Diabetes in the ovaries
Women with polycystic ovarian syndrome (PCOS) produce excessive amounts of androgens, male hormones that cause a cascade of ill effects. When coupled with diabetes (as often happens) the risk of mortality is added to an already serious condition.

PCOS, which affects 6% to 15% of people worldwide, is the most prevalent endocrine condition in women of reproductive age. Although the precise aetiology of PCOS is unknown, it is believed to be brought on by a mix of hereditary and environmental factors. Genome-wide association studies and familial clustering provide initial evidence that PCOS has genetic origins.
However, like many other chronic diseases such as type 2 diabetes mellitus (T2DM), the genetic cause of PCOS is likely polygenic due to the cumulative effect of many genetic variants without a clear pattern of inheritance.
PCOS IN SOUTH AFRICA
It’s nearly impossible to find definitive data on the prevalence of PCOS in South Africa, and the little that exists is out of date. A 2012 study (itself citing a 2007 epidemiological study) in the South African Journal of Obstetrics and Gynaecology noted that anovulation and oligo-ovulation are the two most common causes of female infertility, accounting for about 40% of cases.
PCOS is the predominant factor in this group, affecting about 80% of women with anovulation or oligo-ovulation. Furthermore, a definite diagnosis of PCOS can be made in about 70-80% of women with anovulatory infertility.

PCOS AND DIABETES
Women with PCOS are more likely to experience impaired glucose homeostasis, or abnormal blood sugar levels, in addition to the traditional symptoms of oligo- or amenorrhea (irregular or absent menstrual periods), and clinical or biochemical hyperandrogenism (excess male hormones).
Over time, these conditions may cause prediabetes or T2DM. Around 30% to 40% of patients diagnosed with PCOS are estimated to have prediabetes. Moreover, a significant portion (up to 70%) of PCOS patients with prediabetes may develop T2DM later. Prediabetes greatly elevates the risk of adverse cardiovascular events.
It is inevitable that many women with PCOS should develop T2DM, since both insulin resistance (IR) and beta-cell dysfunction are common in this population. IR is a key player in the underlying pathophysiology of PCOS, and it has been documented in 35% to 80% of women with the syndrome, irrespective of body mass index (BMI).
It should be noted that obesity can further exacerbate IR in women with PCOS. However, beta-cell dysfunction is more common in women with PCOS than in women without the syndrome, even those who are obese.
Unlike PCOS, the prevalence of diabetes in South Africa is well researched, although the current stats are also somewhat out of date. According to the International Diabetes Federation (IDF), an estimated 2.286 million adults in South Africa had diabetes in 2015. This represents a national prevalence of 7.0%, with a range of 3.6% to 14.1%. Of the 2.3 million people with diabetes, 1.4 million (61.1%) were undiagnosed.
ESTABLISHING A PCOS DIAGNOSIS
The Rotterdam Criteria changed the way that PCOS is diagnosed. According to these criteria, any two out of three features of PCOS must be met for a diagnosis to be made (see Figure 1):
- Oligo-amenorrhea (irregular or absent menstrual periods)
- Hyperandrogenism (high levels of androgens)
- Polycystic ovaries (determined by ultrasound)
Hyperandrogenism
Most women with PCOS have high levels of androgens. The ovaries are the main source of these hormones. Testosterone is the most common androgen that is elevated in women with PCOS. It is most often found in the free form, which means that it is not bound to sex hormone binding globulin (SHBG).
SHBG, a crucial protein, binds to sex hormones like testosterone and oestrogen, facilitating their transport in the bloodstream while preventing undesirable tissue binding and subsequent side effects. In cases of PCOS, excessive androgen production by the ovaries can lead to the binding of these androgens to SHBG, diminishing the availability of SHBG for other hormone binding.
Insulin resistance, a condition where the body's cells don't respond adequately to insulin, hinders glucose utilisation for energy. This results in elevated blood glucose levels, which can also suppress SHBG production. The diminished SHBG levels in women with PCOS can result in various issues, including:
- Elevated levels of free testosterone, leading to acne, facial and body hair growth, and irregular menstrual cycles
- Decreased bone density, raising the risk of osteoporosis
- Increased susceptibility to heart disease and stroke.
It is difficult to measure androgen levels in women. The tests that are available are often not accurate at the low levels that are seen in women. The levels of testosterone also vary throughout the day, and other hormones can interfere with the tests. The best way to measure androgen levels in women with PCOS is to use a calculated measure of free testosterone or a high-quality assay that measures total and free testosterone.
Polycystic ovaries
The traditional criteria for ovarian morphology in PCOS state that a woman has PCOS if she has 10 or more follicles that are 2-8 mm in size in one cross-section of the ovary on transabdominal ultrasound. In recent years, there has been some debate about the accuracy of the traditional criteria for ovarian morphology in PCOS. Some studies have found that the traditional criteria are too sensitive, meaning that they identify many women as having PCOS who do not actually have the condition.
The most recent guidelines for the diagnosis of PCOS recommend that ovarian morphology be defined as either 20 or more follicles per ovary and/or an ovarian volume of 10 cm3 or more on either ovary. These criteria are based on studies that have shown that they are more accurate in identifying women who have PCOS.
Oligo-amenorrhea
The average menstrual cycle in adults lasts 28 days, with a normal range of 21-35 days. However, there can be significant variation in cycle length, even among women with regular ovulation. Women with PCOS often have irregular menstrual cycles, with periods that may be too long, too short, too close together or entirely absent.
Irregular menstrual cycles can be a sign of ovulatory dysfunction, which can lead to problems with fertility, as well as weight gain, acne, and hair loss. Most of the current guidelines for the diagnosis of PCOS recommend that irregular menstrual cycles be used as a marker for ovulatory dysfunction.
It is important to note that irregular menstrual cycles can also be caused by other conditions, such as stress, thyroid problems, or eating disorders. Differential diagnosis is therefore required. Generally, serum progesterone levels should be above 3 ng/mL on day 21 of the menstrual cycle to confirm ovulation. If serum progesterone levels are low, ovulation may not be occurring. In this case, other tests, such as luteinising hormone testing, may be helpful.

Image/Shutterstock.com
Image/Shutterstock.com
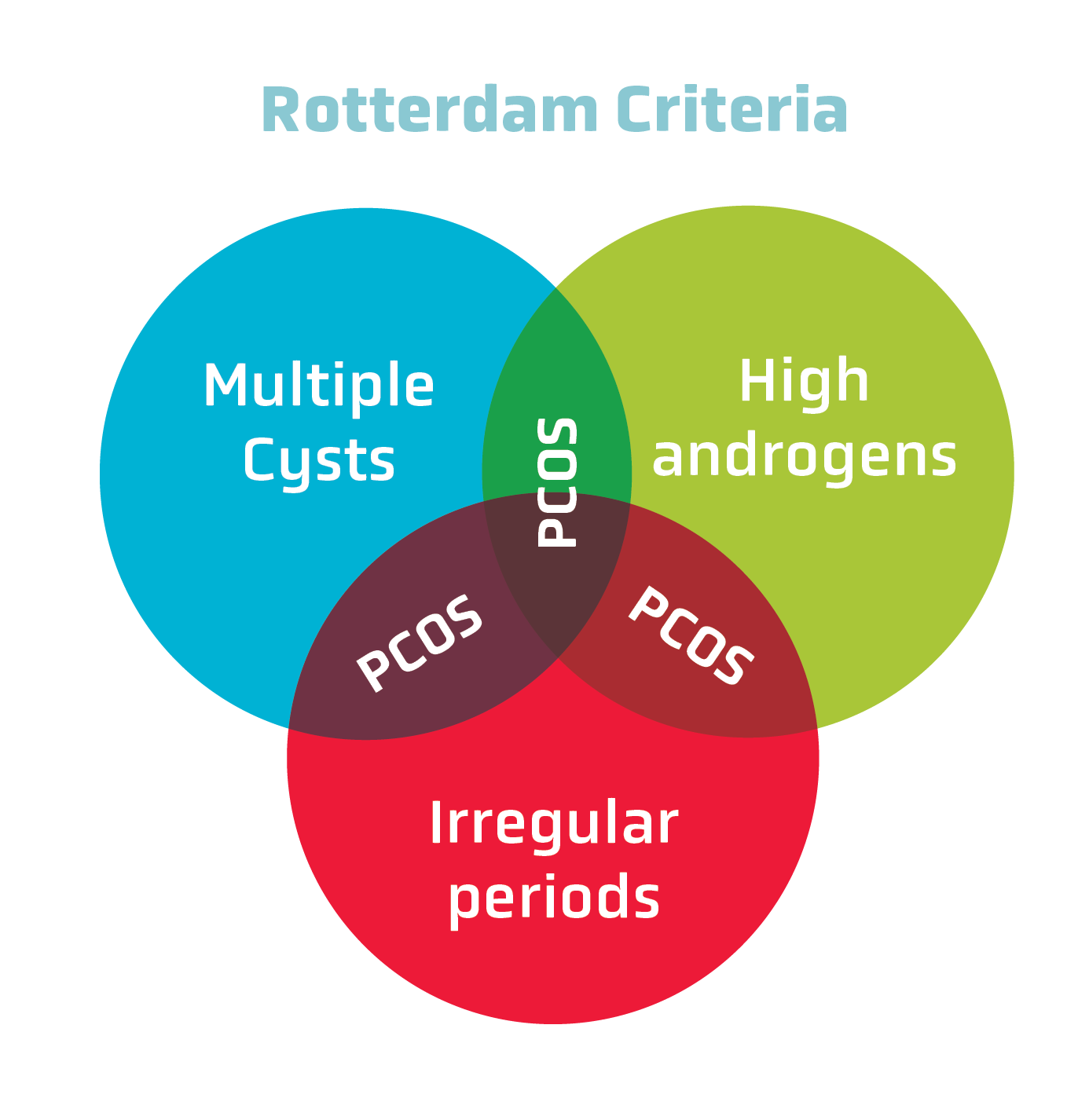
Figure 1: According to the Rotterdam Criteria, two out of three diagnostic conditions must be met to make a PCOS diagnosis.
Figure 1: According to the Rotterdam Criteria, two out of three diagnostic conditions must be met to make a PCOS diagnosis.
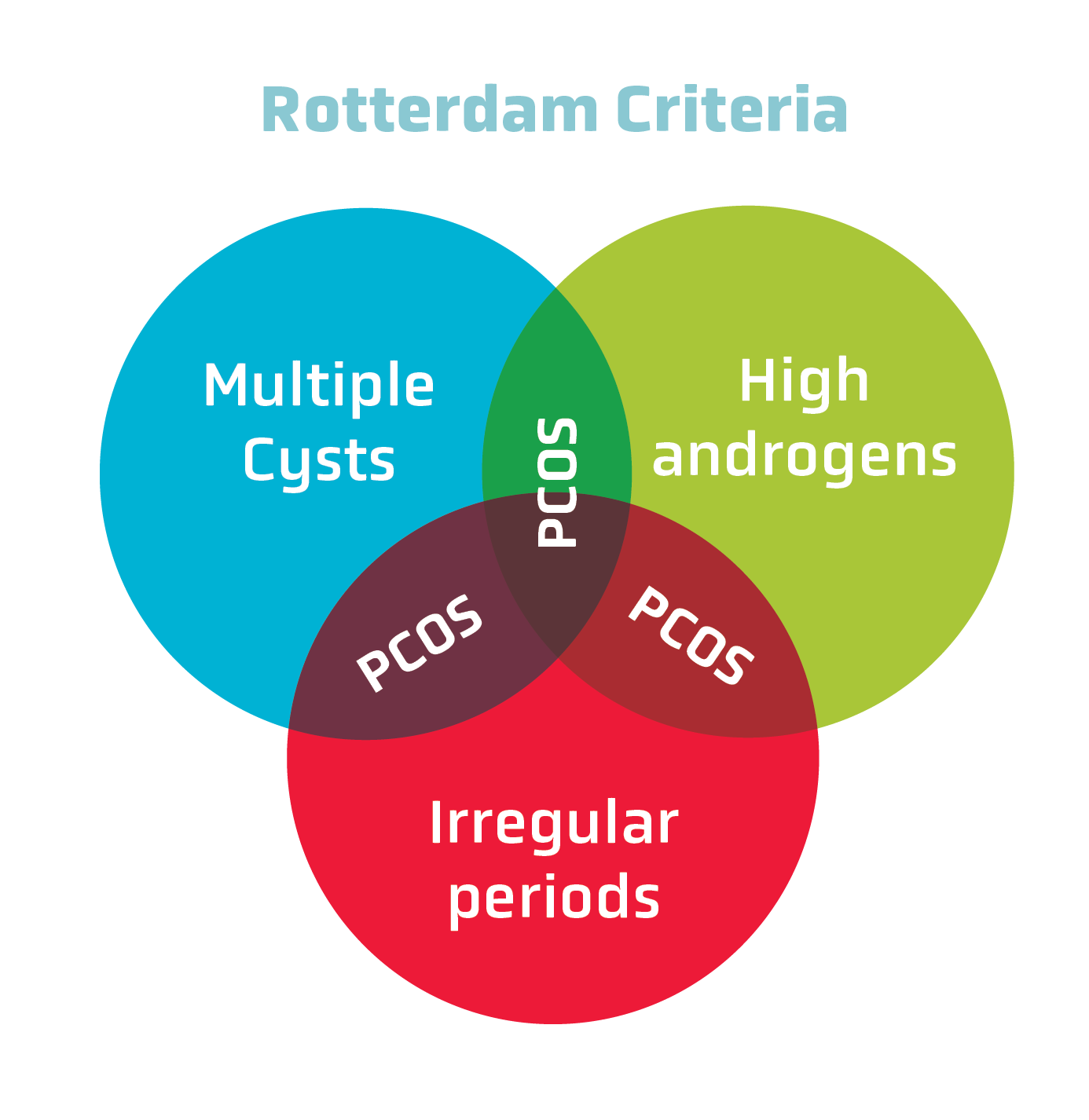
Figure 1: According to the Rotterdam Criteria, two out of three diagnostic conditions must be met to make a PCOS diagnosis.
Figure 1: According to the Rotterdam Criteria, two out of three diagnostic conditions must be met to make a PCOS diagnosis.
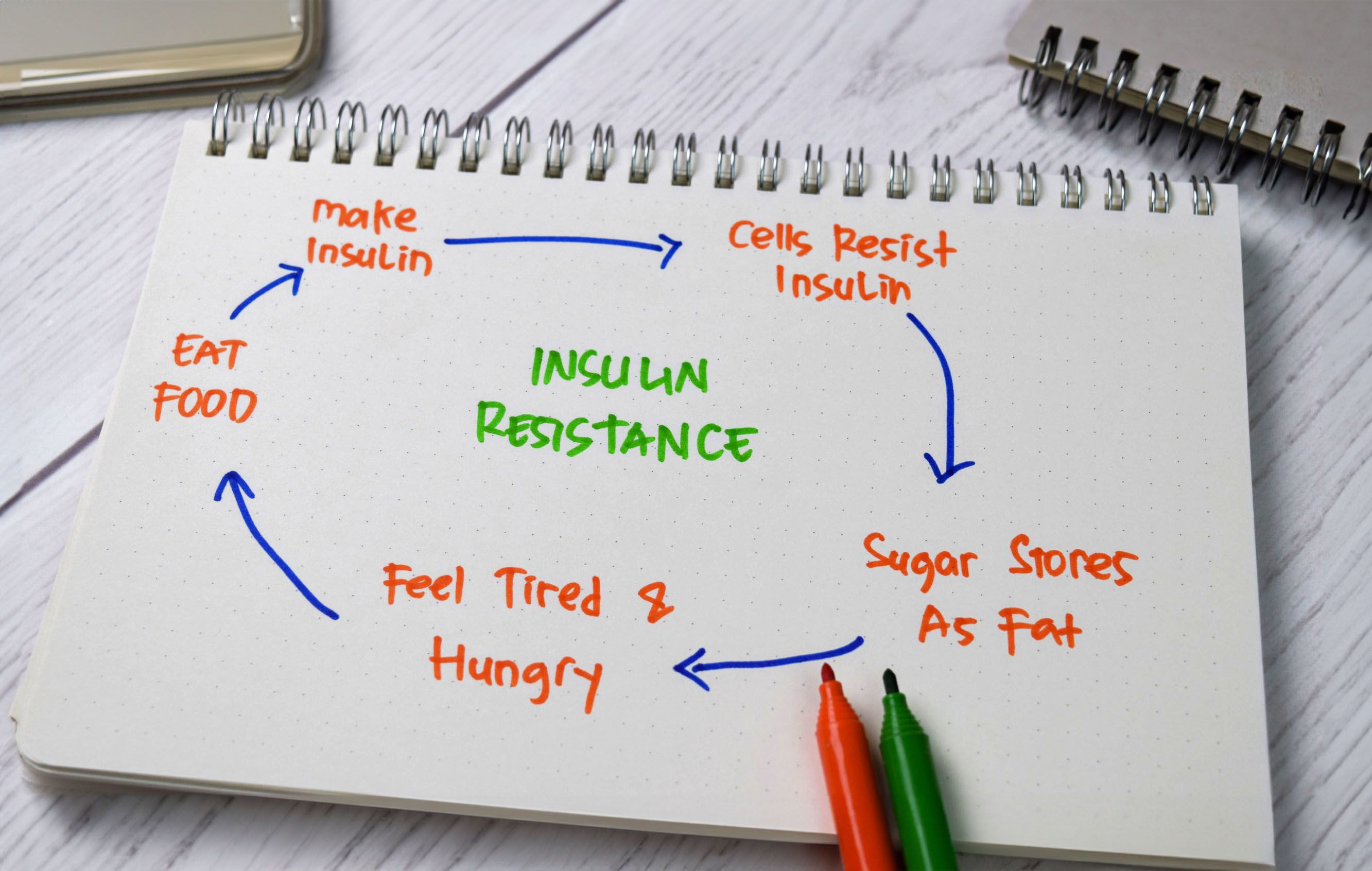
ROLE OF INSULIN RESISTANCE IN PCOS
Insulin resistance is characterised by impaired cellular response to insulin, which hinders glucose transportation into cells and results in elevated blood sugar levels. Researchers are still unsure about the exact cause of insulin resistance in women with PCOS. It is presumed to be influenced by genetics, obesity, and inflammation, but this has not been determined conclusively.
The relationship between PCOS and insulin resistance very much resembles the chicken and the egg. On one hand, insulin resistance in PCOS prompts increased lipolysis, leading to the accumulation of non-esterified fatty acids in the liver and skeletal muscles. Activation of the diacylglycerol/protein kinase C axis then inhibits the insulin receptor, impairing insulin signalling, and reducing glucose uptake in the liver and skeletal muscles, thereby contributing to elevated blood sugar levels. On the other hand, insulin resistance promotes excessive androgen production in the ovaries, contributing to PCOS symptoms.
The assessment of insulin resistance in clinical practice is challenging due to the wide variability in insulin sensitivity among individuals. The gold standard method, the hyperinsulinaemic euglycaemic clamp, is not feasible for routine clinical use. The HOMA-IR test is commonly used in research but is less accurate than the clamp and is more useful for diagnosing insulin resistance than ruling it out. Surrogate indicators of insulin resistance, such as fasting insulin and glucose levels, are influenced by factors other than insulin sensitivity and glucose levels, such as obesity, age, androgen levels, and metabolism.
LIFESTYLE MANAGEMENT
Many clinical guidelines recommend that women with PCOS who have reduced insulin sensitivity should immediately start lifestyle modifications and insulin sensitivity treatment, regardless of their glucose tolerance. However, the primary approach to address insulin resistance is lifestyle modification, which is essential for improving a variety of endocrine and metabolic abnormalities.
Lifestyle modification can be achieved through dietary and exercise prescriptions. The Mediterranean diet, which is rich in vegetables, fruits, seafood, legumes, nuts, whole grains, and vegetable oils, has been shown to be effective in improving endocrine profiles and menstrual regularity in overweight women with PCOS. At least 150 minutes (including over 90 minutes of vigorous activity) of weekly aerobic exercise is ideal, particularly for overweight or obese women.
There is evidence that combining exercise with dietary, or drug interventions is more effective than standalone interventions. In both the general population and among women with PCOS, inadequate and disrupted sleep consistently correlate with heightened risks of obesity, insulin resistance, type 2 diabetes, and cardiovascular disease. Obstructive sleep apnoea and sleep-disordered breathing exacerbate insulin resistance and metabolic consequences linked to abnormal glucose tolerance.
Sleep optimisation is essential for fostering healthy lifestyle changes in PCOS, as sleep influences cortisol levels, appetite hormones, weight management, and cognitive function. Coach your patients to:
- Prioritise regular sleep patterns.
- Create relaxing bedtime routines.
- Avoid stimulants before sleep.
- Ensure an optimal sleep environment.

INSULIN SENSITISERS
Metformin
Metformin is the most widely used insulin sensitiser in PCOS due to its efficacy and safety. It is often used in combination with combined oral contraceptives (COCs), especially for overweight or obese patients. Metformin has been shown to have beneficial effects on the pathogenetic mechanisms underlying PCOS, restoring ovarian function and improving metabolic profile, especially insulin sensitivity. It also has anti-inflammatory effects, which can regulate T-cell balance, and performs beneficial anti-inflammatory actions by influencing mitochondrial function and modulation of immunity.
Inositol
In addition to metformin, inositol supplementation has also been shown to be beneficial in PCOS. Myo-inositol (MYO) and D-chiro-inositol (DCI) are the most abundant forms of inositol present in humans. MYO can be converted to DCI by an epimerase enzyme, stimulated by insulin. The DCI/MYO ratio is regulated by every organ and tissue, and is involved in several physiological processes, such as ion channel permeability, cytoskeleton remodeling, developmental processes, stress response, and endocrine signal transduction.
Studies have confirmed the role of MYO and DCI as insulin sensitisers, improving both metabolic and oxidative imbalance. In addition, MYO also acts as a secondary messenger of FSH signaling in the ovary. Its use as a supplement can restore regular menses and ovulation, improve oocyte and embryo quality, and reduce the amount of recombinant FSH administered during ovarian stimulation protocols. On the contrary, the supplementation of DCI alone may worsen the insulin-mediated androgen synthesis and fertility in PCOS women.
Subsequent studies have demonstrated that the combination of MYO and DCI in a 40:1 ratio is the most similar to the plasma ratio reported in healthy women and is the most effective at improving ovarian function and metabolic profile in PCOS patients. Finally, the association of MYO and/or DCI with metformin and/or COCs could have a synergistic action and reduce adverse effects.
α-lipoic acid and myo-inositol
Recently, α-lipoic acid (ALA) has become noted as a potential therapeutic for polycystic ovary syndrome (PCOS) and insulin resistance. ALA is a powerful antioxidant that can scavenge reactive oxygen species (ROS) and regenerate other antioxidants. It also inhibits the inflammatory pattern mediated by nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and has an immunomodulatory function.
In the metabolic field, ALA can improve insulin sensitivity by activating the expression of 5′-adenosine monophosphate-activated protein kinase (AMPK). AMPK is a cellular energy sensor that induces the translocation of glucose transporter 4 (GLUT4) to the plasma membrane with an insulin-independent mechanism. A reduced ALA synthesis, probably due to the downregulation of the lipoic acid synthase (LASY) that occurs during T2DM and insulin resistance, is thought to affect the normal glucose uptake and utilization in skeletal muscle cells.
In addition to its antioxidant and metabolic effects, ALA has also been shown to have anti-inflammatory and immunomodulatory effects. This makes it a promising therapeutic agent for PCOS, which is characterised by chronic inflammation and insulin resistance. In therapeutic practice, ALA is often combined with MYO for a variety of reasons.
In a study investigating the effects of treatment with MYO on circulating insulin, glucose tolerance, ovulation, and serum androgens concentrations in women with PCOS, MYO was discovered to have significant benefits. When comparing MYO to placebo in women with PCOS, it was determined that:
- MYO led to decreased insulin levels, improved glucose tolerance, and enhanced insulin sensitivity
- Promoted ovulation, reducing serum total testosterone by 65% and serum free testosterone by 72%
- MYO was associated with a 36% decline in post-meal insulin release, a 15% improvement in post-meal glucose utilisation, and an 84% rise in insulin sensitivity.
A study by Okanovic et al focused on obese patients with T2DM explored ALA's impact on weight, lipid status, and blood glucose regulation. Those treated with ALA and metformin exhibited significantly lower triglyceride levels compared to the metformin-only group (p<0.05), while cholesterol concentration remained unchanged. However, glucose concentration did not differ between groups after ALA treatment. Overall, a daily ALA dose of 600 mg effectively regulated weight loss and triglyceride levels in obese patients with type 2 diabetes, suggesting ALA's potential as an additional treatment for these patients.
In a study by Cappelli, et al, which compared two treatments for women with insulin-resistant PCOS, namely high-dose metformin alone and a combination of lower-dose metformin with MYO and ALA, the goal was to assess differences in metabolic and endocrine parameters between the two groups, as well as side effects.
Forty-five women were initially enrolled, with 40 completing the trial. Participants were of reproductive age, obese, and had insulin resistant PCOS. It was found that both treatments were effective in improving various parameters, but that the metformin/ALA/MYO combination (Group B) showed more significant results and had fewer side effects.
Group B had better overall outcomes in terms of insulin resistance, progesterone levels, androgen levels, and hirsutism severity. This suggests that metformin/ALA/MYO combination is a promising therapeutic option for obese women with PCOS who prefer non-hormonal treatments and want to minimise the risk of serious side effects.
CONCLUSION
Our knowledge of PCOS is still incomplete, but our understanding of therapeutics that affect this condition has steadily increased. The association between PCOS and T2DM is also poorly understood, but highly effective treatments strategies exist. While lifestyle management remains primary, it is best combined with treatment incorporating insulin sensitisers like metformin and MYO. When combined with ALA, MYO delivers excellent therapeutic benefits by regulating and reducing inflammation and insulin in a way that ameliorates the effects of T2DM and PCOS simultaneously.
REFERENCES
Armanini, D, et al, 2022. Controversies in the Pathogenesis, Diagnosis and Treatment of PCOS: Focus on Insulin Resistance, Inflammation, and Hyperandrogenism. International Journal of Molecular Sciences. 2022; 23(8):4110.
Condorelli, RA, et al, 2017. PCOS and diabetes mellitus: from insulin resistance to altered beta pancreatic function, a link in evolution. Gynecological Endocrinology, 33:9, 665-667.
Cowan, S, et al, 2023. Lifestyle management in polycystic ovary syndrome – beyond diet and physical activity. BMC Endocrine Disorders volume 23, Article Number: 14.
Fruzzetti, F, et al, 2020. Long-term treatment with α-lipoic acid and myo-inositol positively affects clinical and metabolic features of polycystic ovary syndrome. Gynecological Endocrinology, 36:2, 152-155.
Guarano, A, et al, 2023. Alpha Lipoic Acid Efficacy in PCOS Treatment: What Is the Truth? Nutrients. 2023; 15(14):3209.
Livadas, S, et al, 2022. Polycystic ovary syndrome and type 2 diabetes mellitus: A state-of-the-art review. World J Diabetes. 2022 Jan 15; 13(1): 5–26.
Moghetti, P, et al, 2020. Insulin resistance and PCOS: chicken or egg? Journal of Endocrinological Investigation, Volume 44, Pages 233–244.